English page
Kanefumi KITAHARA – Professor, Applied Starch Chemistry
Kiyotaka FUJITA – Associate Professor, Carbohydrate-related Enzymology
Our research interests relate to the basic and applied chemistry of carbohydrate resources and the carbohydrate-related enzymes. Here, we briefly introduce two of our recent research topics: (1) Novel sweetpotato starch having unique properties, and (2) exploration of the metabolic pathway of indigestible carbohydrates by intestinal bacteria inhuman colons.
Sweetpotato is one of the important starch resources in the tropics and warm regions of the world. In Japan, most of sweetpotatoes are produced in Kagoshima. In order to promote the sweetpotato utilization, we have collaborated with a research institute of the National Agriculture and Food Research Organization of Japan to develop sweetpotato breed lines having unique starch properties. As a result of effort, we found a promising sweetpotato line whose starch paste showed very slower retrogradation (process of gelatinization of starch) compared to the starches of ordinary sweetpotatoes.
We found that the unique starch of the newly developed sweetpotato had other interesting properties, such as low gelatinization temperature and high susceptibility to chemical and enzymatic degradation. These technological advantages of the newly developed starch offer high potential for its applications in food industry and are expect to promote the sweetpotato utilization as well as activation of the regional economy.
New sweetpotato starch having a slow retrogradation
Bifidobacterium longum, an intestinal bacterium, metabolizes human-indigestible carbohydrates as carbon sources in human colons. For example, type II arabinogalactans (type II AGs) and β-L-arabinooligosaccharides (β-AOSs), hydroxyproline (Hyp)-linked sugar chains of Hyp-rich glycoproteins (HRGPs) including arabinogalactan-proteins (AGPs) and extensin found in plant cell walls, cannot be digested by humans. Despite the broad distribution of type II AGs and β-AOSs in plants kingdom, their degradative enzymes are yet to be found. Recently, we cloned and characterized the degradative enzymes for the type II AGs and β-AOSs from B. longum. The enzymes are exo-β-1,3-galactanase, exo-β-1,6-galactobiohydrolase, α-L-arabinofuranosidase, β-L-arabinobiosidase, and β-L-arabinofuranosidase. These enzymes are encoded in conserved gene clusters on several B. longum genomes, but not in other intestinal bacteria, which implies a unique strategy of B. longum for acquiring carbon sources in human colons. We work to explore the whole metabolic pathway of the plant glycoproteins and polysaccharides in B. longum. The study would contribute to the understanding of the molecular bases of prebiotics and probiotics in human health.
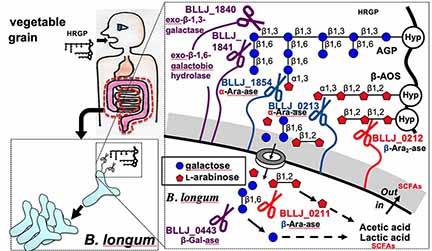
HRGPs are prebiotic glycoproteins for B. longum